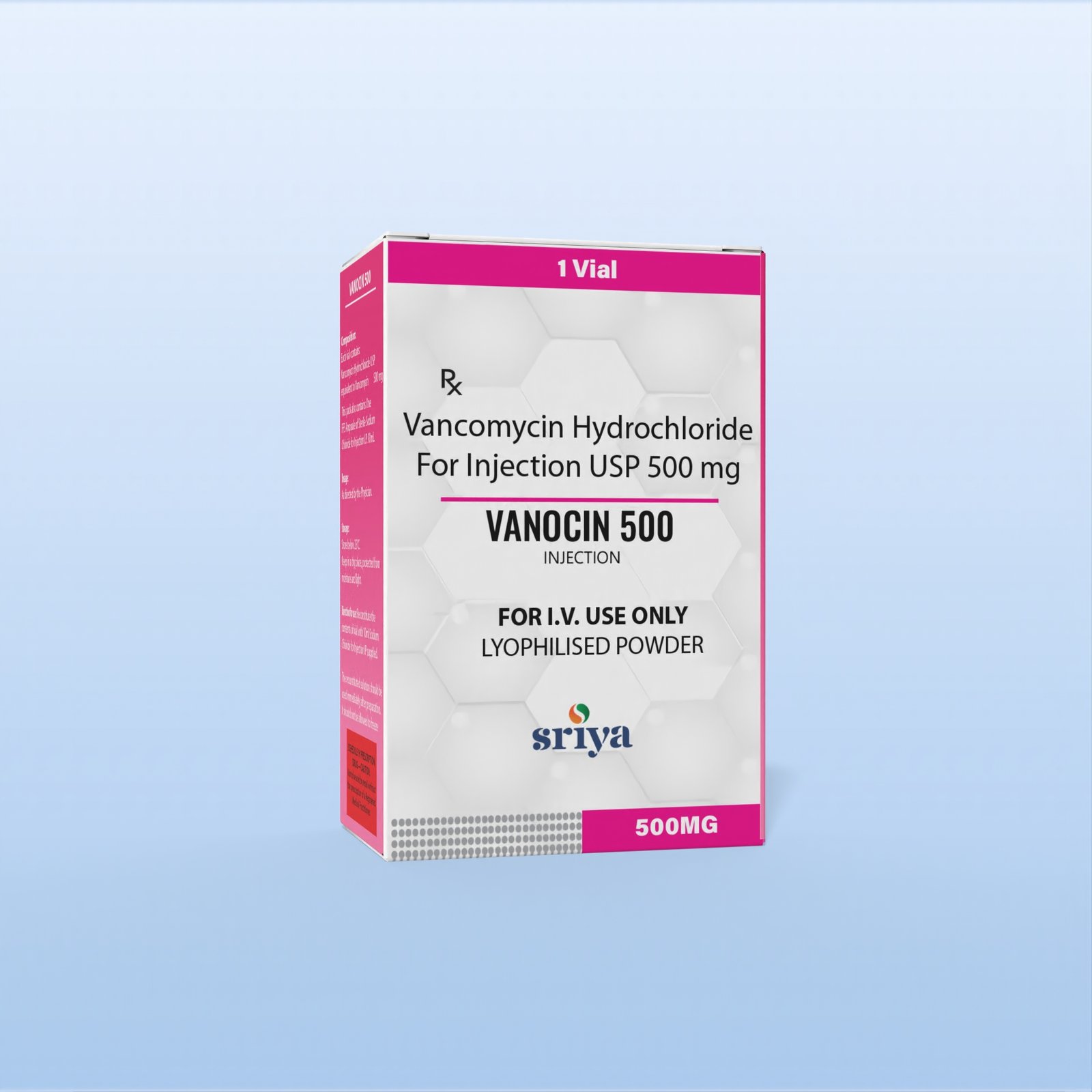
Vancomycin Hydrochloride Injection
|
Generic Name |
Vancomycin Hydrochloride |
|---|---|
|
Strength |
500 mg / 1 gm |
|
Form |
Injection |
|
Packaging |
Vial |
|
Therapeutic use |
Antibiotic |
|
Manufacturing Period |
30 to 45 Days |
Available Composition of
| Name | Strength | Therapeutic use | View More |
|---|---|---|---|
| Vancomycin Hydrochloride Injection | 1 gm, 500 mg | Antibiotic |
Product Description For Vancomycin Hydrochloride Injection
products details and uses
Vancomycin Hydrochloride Exporter India, Manufacturer, Wholesaler & Distributor
Vancomycin Hydrochloride for Injection, USP/BP/EP, sterile lyophilized powder in 500 mg and 1 g vials. Glycopeptide antibiotic indicated for severe Gram-positive infections, including MRSA, septicemia, endocarditis, osteomyelitis, and pneumonia, especially in beta-lactam–resistant cases. Acts by inhibiting bacterial cell wall synthesis, leading to cell death. For IV infusion only after reconstitution. Supplied in single-dose vials with carton and insert. Schedule H prescription drug.
Global Distribution Network
With a strong logistics and compliance framework, we successfully export Cefotaxime to a broad range of international markets, including: Venezuela, Brazil, Cambodia, Philippines, Nigeria, Myanmar, Thailand, Singapore, Russia, Kyrgyzstan, Tajikistan, Kenya, Zimbabwe, Somalia, Djibouti, Belarus, Macedonian, And Turkey.